Background
Immune reconstitution (IR) after allogeneic hematopoietic stem cell transplantation (HSCT) is crucial to avoid infection, relapse, and graft versus host disease (GvHD). Torque Teno Virus (TTV) is a highly prevalent and ubiquitous small DNA virus which constitutes the main component of human virome. In the transplant setting, TTV viral load (VL) seems to correlate with immunodeficiency status, but most studies have focused on solid organ transplant recipients.
Aims
To study the kinetics of TTV VL in blood (log10 copies/ml), and its presence in nasopharyngeal aspirate (NPA) by qualitative PCR in children undergoing HSCT, and to study their correlation with HSCT outcomes and other markers of IR in the first year after the procedure.
Methods
Prospective single center study including patients <18 years old who underwent HSCT in 2020-2022, excluding those with lung disease. Blood and NPA samples were collected pre-HSCT and at days 0, +15, +30, +90, +180, +270 and +360 post-HSCT. Immune cell typing including T/B/NK, and NK phenotyping and cytotoxicity (%CD107a+) were performed in blood, and T/NK cells by flow cytometry and respiratory viruses by multiplex PCR were tested in NPA. TTV in blood was analyzed with TTV R GENE® kits (Biomérieux, France), whereas for NPA an in house PCR developed at the National Centre for Microbiology was used.
Results
We included 30 patients, whose main characteristics are summarized in Table 1. The most frequent indication for HSCT was malignant disease (76.7%). Donor was mainly HLA mismatch (67%). All patients had engraftment (median 10 days, IQR 10-13.5), but one experienced secondary graft failure. Acute GvHD occurred in 73% of patients (63% grades II-IV), and chronic in 37% (10% severe). Almost all patients (93%) presented at least one episode of infection (median 3, IQR 1-6). Endothelial injury complications occurred in 17%. During the first year post-HSCT, 2/21 patients relapsed. Overall survival was 67% (median follow up 601 days, IQR 424.25-880).
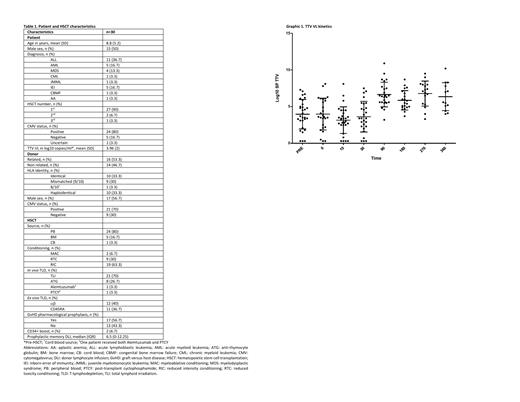
No significant differences were found in pre HSCT TTV VL regarding patient age, sex, underlying disease, nor CMV status. TTV VL (Graphic 1) decreased after conditioning to minimum at +15 (mean log10 3.149, SD 1.8), increased from +30 being highest at +270 (mean log10 6.778, SD 1.7), and at +360 was still higher than pre-HSCT (mean log10 6.358, SD 1.9). At day +15, each increase in log10 TTV VL increased the risk of dying 39% (p=0.027). At day +30, TTV VL was higher when total lymphoid irradiation (TLI) was used vs serotherapy (p=0.01). No differences were found regarding GvHD development or infections episodes.
TTV could be detected in NPA, although its prevalence seems lower than in blood: 14/44 NPA samples resulted positive (32%), most at day +15 (36%); while 50/182 (27.5%) were positive for respiratory virus, the majority at +270 (28%), without identifying any association with TTV positivity.
We found several associations between TTV VL and some parameters of IR: there was an inverse correlation with leukocytes, total, and B lymphocytes at +90 (p=0.001, 0.013, and 0.035 respectively), and with leukocytes at +270 (p=0.042). There was also an inverse correlation at +90 with %CD4+CD45RA+ (p=0.043), and direct with %CD4+CD45RO+ (p=0.038). Finally, TTV VL was direclty associated at pre-HSCT with %CD3+HLADR+ (p=0.035).
In our series, associations between TTV VL and NK were positive, the stronger with %CD16+ at +270 (p=0.023). Nevertheless, an inverse correlation was found with %CD107a+ at baseline (p=0.04) and at +15 (p=0.013). There was a lack of a consistent pattern of expression of NK receptors along our study.
Conclusions
1. TTV is usually detected in blood in children undergoing HSCT, whereas its detection in NPA is less frequent.
2. TTV could be a potential early biomarker of survival after pediatric HSCT.
3. TTV correlates with less NK citotoxicity before and early after HSCT.
4. TTV VL correlates with slower IR at day 90 after HSCT.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal